Department of Research Support
In cooperation with other organizations and agencies, the Department of Research Support supports the development and practical applications of drugs, medical devices, and products for regenerative medicine and other therapies.
To effectively advance the development of pharmaceutical and medical products, staff members with high-level expertise provide guidance and suggestions based on their advisory experience.
Promotion of Development of Drugs and Other Products
In order to effectively promote the development of drugs, medical devices, and products for regenerative medicine and other purposes, it is necessary to facilitate mutual collaboration (including national testing and research institutes) among universities, industries, and public organizations, according to the stage of development.
Therefore, the Department of Research Support provides instruction, advice, and other types of support by the program officers (POs) to accelerate the practical application of drugs and other products, taking advantage of its expertise and experience of development of drugs and other products.
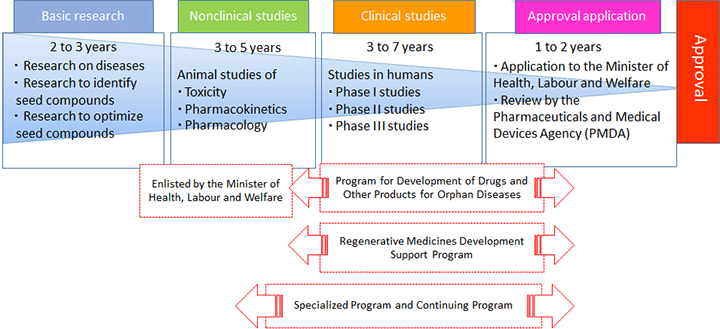
Orphan Products Development Support Program
R&D efforts for drugs and medical devices are time- and resource-intensive. In particular, many R&D programs aimed at developing therapies for intractable diseases do not make sufficient progress because, despite their high unmet medical needs, the small populations of patients make it difficult for manufacturers to recoup their financial investments.
To address this problem, a government-led support initiative was launched in 1993. This initiative provides special support for R&D of pharmaceutical and medical product candidates that have been granted orphan status by the Minister of Health, Labour and Welfare (MHLW Minister). The Act for Partial Amendment to the Pharmaceutical Affairs Act, etc. enacted in 2013 (Act No. 84/2013) introduced a new legal term, "regenerative medicine (cellular and tissue-based) products," to medical and pharmaceutical regulations. At the same time, a new initiative has been started concerning "regenerative medicine (cellular and tissue-based) products for orphan diseases."
This program aims to deliver new, safe, and effective orphan drugs, orphan medical devices, and regenerative medicine (cellular and tissue-based) products for orphan diseases into clinical practice at the earliest opportunity. To achieve this goal, NIBIOHN offers financial support for the development of orphan-designated products, and also provides appropriate guidance and advice for obtaining marketing authorization.
References: Criteria for Orphan Designation by the MHLW Minister (Pharmaceutical and Food Safety Bureau Notification No. 0401-11)
(1) Number of patients
The number of patients who may use the drugs, medical device or regenerative medicine should be less than 50,000 in Japan.
(2) Medical needs
The drugs, medical devices or regenerative medicine should be indicated for the treatment of serious diseases, including difficult-to-treat diseases. In addition, they must be drugs, medical devices or regenerative medicine for which there are high medical needs satisfying one of the following criteria.
- There is no appropriate alternative drug/medical device/regenerative medicine or treatment
- High efficacy or safety is expected compared with existing products
(3) Possibility of development
There should be a theoretical rationale for the use of the product for the target disease, and the development plan should be appropriate.
Regenerative Medicines Development Support Program
The Act for Partial Amendment to the Pharmaceutical Affairs Act, etc. enacted in 2013 (Act No. 84/2013) introduced a new legal term, "regenerative medicine (cellular and tissue-based) products." In addition, this law established that if regenerative medicine (cellular and tissue-based) products can reasonably be expected to have clinical validity, they can be approved for marketing under the terms and conditions necessary to ensure their appropriate use.
To fill the gap in unmet medical needs in patients with rare diseases or refractory disorders, this program seeks to ensure timely commercialization of regenerative medicine (cellular and tissue-based) products. Since FY 2014, NIBIOHN has started to grant financial assistance to projects focusing on practical applications of candidate products (i.e., use in clinical settings).
Specialized Program and Continuing Program
Specialized Program
In order to support venture and other businesses engaging in development of drugs and medical devices at the stage of practical application, we provide supports in various forms. For example, we provide instruction and advice to promote commercialization of products developed in research projects approved as "Projects suited for practical application," for which a grant was provided from fiscal year (FY) 2004 to FY 2010. So far, one product has been approved, and two approval applications have been filed.
Continuing Program
To promote research and development of drugs, medical devices, and other products based on cutting-edge technologies in the private sector, we assist corporate bodies that are conducting research and development funded by multiple companies. To this end, we provide support, including instruction and advice, to relevant companies that receive funding from the Organization for Pharmaceutical Safety and Research (current Pharmaceuticals and Medical Devices Agency) from FY 1987 to FY 2003. Some of the products developed with our support have been launched and continue to be developed by the licensees.
