Laboratory of Drug Discovery Resources Research(JCRB Cell Bank)
1. Key members
| Project Leader | Arihiro Kohara |
|---|---|
| Postdoctoral associates | Junpei Inui |
| Technical assistant | Setsuko Shioda, Azusa Ohtani, Midori Ozawa, Noriko Hirayama, Eiko Kawaguchi, Naomi Hara, Masashi Iemura, Hiromi Ikeda, Junko Sakai,Kumi Takagi,Meguni Wada,Nahoko Kotani, Kikuko Ietsuka, Tomoko Tano, Sayaka Miyata, Aya Ohnishi, Nozomi Sugihara |
| Collaborating Researcher | Motonobu Satoh, Kenzo Banba |
2. Background and objectives
The JCRB Cell Bank was originally established in National Institute of Health, Tokyo in 1984. In 2005, the Cell Bank has relocated to the NIBIO, Osaka. The cultured cells represent an in vitro model for numerous studies in medical science and pharmaceutical research. In particular, human-derived cultured cells are indispensable for these studies. As the JCRB cell bank, we are collecting human culture cells derived from various disease patients, and human stem cells including induced pluripotent stem (iPS) cells and adult mesenchymal stem cells. The cells are checked by several strict tests, and then the qualified cells are provided to researchers. Further, we are developing the qualification method, functional evaluation method, and also culture method for human cells in order to support fundamental research on medical or pharmaceutical sciences.
3. Overview of our research
I. JCRB Cell Bank: From the collection to distribution of cells and information
We are collecting human culture cells derived from various disease patients, and human stem cells including induced pluripotent stem (iPS) cells and adult mesenchymal stem cells. The cells are qualified by testing for microbial contamination, virus contamination, and cross culture contamination, and also confirming their karyotype and cell characteristics. And then the qualified cells are provided to researchers. The information of these cells is available at our website, http://cellbank.nibiohn.go.jp/english/

II. Development of quality control procedures and characterization methods for cultured cells
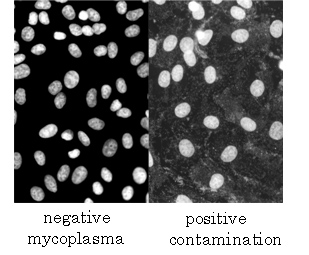
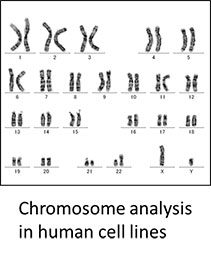
In the cell culture area, mycoplasma contamination, virus contamination and cross contamination with other cell lines are serious problems to be sorted out. We are developing the procedures for testing for microbial contamination, virus contamination, and cross culture contamination. We are also developing characterization methods for culture cell lines. In particular performs a detailed analysis of the genome as a representative of the chromosome analysis of cell lines, it has put a force on the acquisition of the cell information.
III.Development of new cell lines for drug discovery
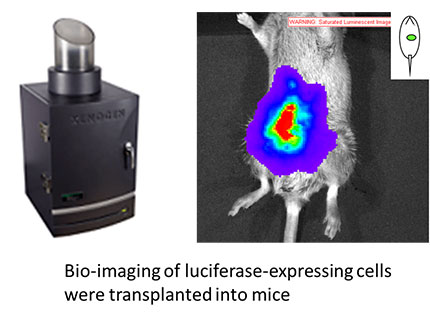
Cell lines have been developed as a tool that cannot be essential in drug discovery research. However, the recent genetic modification techniques, improvement of additional tools have been developed. In particular, we are promoting the development of cell lines useful in drug discovery, such as immortalized cells and luciferase expressing cells.
Laboratory of Cell Cultures (JCRB Cell Bank)
| Website | https://cellbank.nibiohn.go.jp/english/ |
|---|---|
| jcrb-cell*nibiohn.go.jp (replace * by @) |
