HOME › Research and Resources › Research on Disease Bioresources › Laboratory of Rare Disease Biospecimen (Rare Disease Bank)

Laboratory of Rare Disease Biospecimen (Rare Disease Bank)
1. Key members
- Project leader - Arihiro Kohara
- Researcher - Ryuichi Sakate
- Specially Appointed Researcher - Makoto Hirata
- Technical assistant - Mayako Tada, Akiko Hinomura, Sanae Tanaka
2. Background and objectives
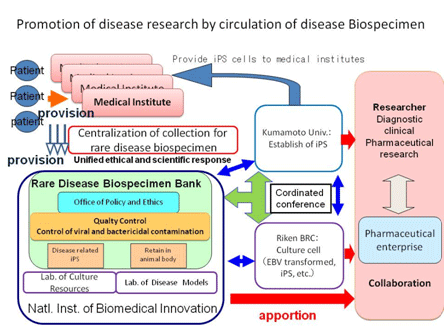
It is important to improve research infrastructures and to plan for research revitalization in order to overcome rare diseases. To that end, it is essential that we construct a better system where we comprehensively collect and store the valuable biological specimens obtained with patient consent as research resources, and allow those researchers engaged in disease research and drug development research to make broad use of those specimens. The objective of this research is the improvement of the quality of the research resources for rare diseases, the operation of the "Laboratory of Rare Diseases Biospecimen" (the "Rare Diseases Bank") that facilitates the distribution of those resources and the provision of rigorously quality-controlled specimens to contribute towards the efficient implementation of research into overcoming rare diseases.
3. Overview of our research
In order to further promote research into overcoming rare diseases, we have decided that the work of the "rare Diseases Bank" will be conducted centered on the National Institute of Biomedical Innovation. This work is being done in order to contribute towards overcoming 130 diseases designated as rare diseases by the Japanese Ministry of Health, Labour and Welfare. To that end, we are conducting research into the following issues:
I. Provision of an environment for informed patient consent
It is important to comprehensively collect and manage the specimens with patient cooperation, then to apportion those specimens to researchers, thus expediting their research activities. In such cases it is an absolute requirement that patients' consent be obtained when they are providing the specimens, and that their personal information and privacy are securely guarded. It is under these auspices that this work is to be conducted.
II. Coordination with physicians (or medical institutions) in the request for specimens and their use in research
The physicians (or medical institutions) that provide us with clinical specimens are charged with the necessary and important function of diagnosis. We are constructing a system so that when the specimens are to be used in research, it can decide upon the suitability of both the research and oversee that the rights of the physicians of the medical institutions providing the specimens are appropriately upheld, thus ensuring these valuable materials are used reasonably and impartially in research.
III. Implementation of an ethical review
Before the clinical specimens are utilized in research, they must pass through an ethical review conducted by the respective research ethical review boards of the medical institutions where the specimens have been deposited, and the institutions affiliated with the researchers who make use of the rare diseases bank and the specimens. This research, on the topic of medical research ethics, will also obtain the cooperation of the office of policy and ethics research of this institute.
Through impartially apportioning the research specimens based on a set of unified rules, we will contribute towards the promotion of therapies and the development of pharmaceuticals associated with overcoming rare diseases. Our goal is the establishment of a sustained "clinical" and "research" coordination cycle.
This institution will carry on the work of the former Gene Bank organization (the BAC clone screening service, DNA clone apportionment, and provision of the integrated Macaca fascicularis database). For further information about the previous work, please visit the following sites:
Old Gene Bank web site: http://genebank.nibiohn.go.jp/index_e.html
Macaca fascicularis cDNA database (QFbase): http://genebank.nibiohn.go.jp/qfbase/index.html
Chimpanzee cDNA database (PRIGEN): http://www.prigen.org
To inquire about the Laboratory of Rare Disease Biospecimen, please contact
To inquire about DNA Bank, please contact
To inquire about the Rare Disease Biospecimen Bank, please contact



